First Successful Synthesis of Naphthocyclinones Since Their Discovery Fifty Years Ago
Researchers develop an innovative strategy to produce elusive antibiotic compounds with potential medical and biotechnological applications
For the first time, β- and γ-naphthocyclinones were successfully synthesized in a laboratory, report scientists from Science Tokyo. By combining originally developed tactics and innovative strategies, they overcame big challenges in producing these complex molecules, which had eluded synthesis efforts for decades. This breakthrough contributes to our understanding of naphthocyclinone chemistry and opens doors to the development of compounds with potential applications in medicine and biotechnology.
First Successful Synthesis of Naphthocyclinones
Ando et al. (2024) | Angewandte Chemie International Edition | 10.1002/anie.202415108
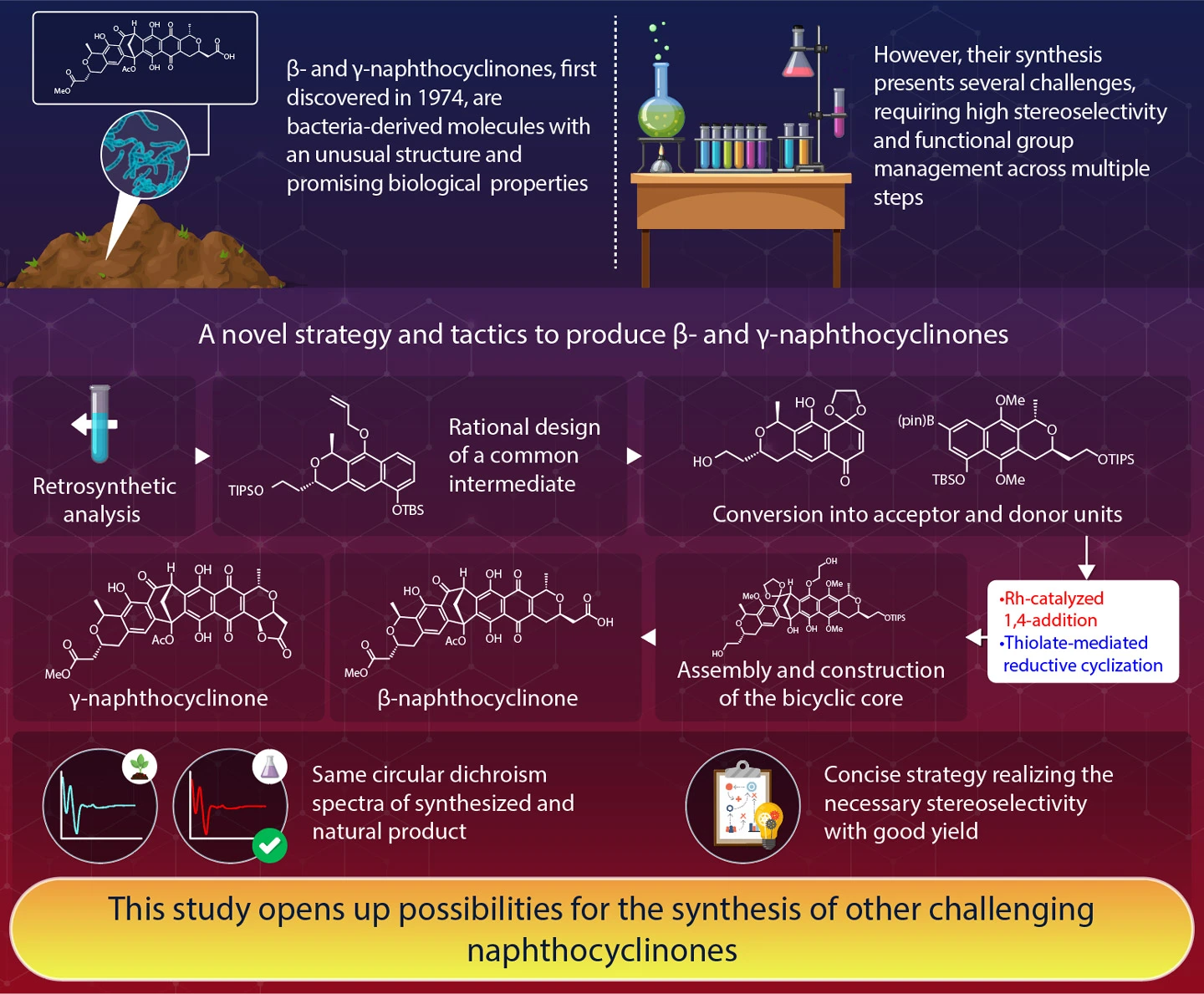
Back in 1974, German researchers discovered peculiar chemical compounds, present as red pigments in soil bacteria from a volcanic crater. These biomolecules, which came to be known as naphthocyclinones, are representative of a family of antibiotics with potential medical or biological applications. Despite their promising properties, artificially synthesizing these naphthocyclinones has proven to be quite challenging.
Fortunately, as reported in a paper published in Angewandte Chemie International Edition on 10th October 2024, scientists from Institute of Science Tokyo (Science Tokyo) successfully accomplished this difficult task. Led by Associate Professor Yoshio Ando, this research team crafted an elegant strategy to synthesize both β- and γ-naphthocyclinones at high yields, opening up the way to further studies of these and related compounds and increasing their availability for practical use.
This unprecedented feat took five decades to complete because of the structure of β- and γ-naphthocyclinones. Both molecules are quite similar and can be seen as the combination of two smaller, independent units referred to as monomers A and B. The bridge that connects these two monomers in the final compounds, however, is a rather complex structure called a bicyclo[3.2.1]octadienone core. Getting this core to form as the monomers bond is difficult, as the position and orientation of the constituents need to be correct while preventing the formation of side products or the modification of functional groups in other parts of the monomers themselves. On top of this, stereoselectivity is necessary when synthesizing the monomers. This means that the reactions must preferentially produce one specific spatial arrangement of atoms in the monomer, with functional groups at the correct orientations.
The research team tackled these challenges using a technique called retrosynthetic analysis. Simply put, they worked backward starting from the final target molecule (β-naphthocyclinone), breaking it down into simpler precursor compounds to identify a feasible synthetic pathway. When designing monomers, the team came up with a two-step approach employing Rh-catalyzed 1,4-addition and thiolate-mediated reductive cyclization that would lead to the bond formations necessary to obtain the bicyclo[3.2.1]octadienone core. To improve the efficiency of their overall strategy, they also designed a common intermediate molecule from which both the designed monomers could be easily derived.
Thanks to their attention to detail and careful consideration of all intermediate reactions, the researchers managed to synthesize β-naphthocyclinone with an acceptable yield of at least 70% at every step. To produce γ-naphthocyclinone, they employed a process called oxidative lactonization to obtain it from β-naphthocyclinone, achieving a yield of 87%. "The circular dichroism spectra of our synthesized compounds were identical to those of naturally occurring ones, implying that the absolute configuration of synthetic and natural molecules was the same," remarks Ando, highlighting their success.
As the first-ever reported method to synthesize these complex molecules, this study provides valuable insights and tools to chemistry researchers. "We demonstrated a concise strategy toward heterodimer synthesis via the logical design of a common intermediate. This approach could unlock the synthesis of other congeners of naphthocyclinones," says Ando. "Further efforts along these lines are already in progress in our laboratory," he adds, with eyes on the future.
With any luck, the insights obtained in this work will pave the way to novel chemicals with medical, environmental, and biological value.
Reference
- Authors:
- Yoshio Ando1*, Taiju Hoshino1, Nozomi Tanaka1, Mark M. Maturi1, Yusuke Nakazawa1, Takumi Fukazawa1, Ken Ohmori1*, and Keisuke Suzuki1
- Title:
- Total Syntheses of β- and γ-Naphthocyclinones
- Journal:
- Angewandte Chemie International Edition
- Affiliations:
- 1 Department of Chemistry, Institute of Science Tokyo, Japan
Related articles
Further information
Associate Professor Yoshio Ando
Department of Chemistry, School of Science, Institute of Science Tokyo
- yando@chem.titech.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@ml.tmd.ac.jp