Achieving Precise Timing for DNA Droplet Division: A Step Towards Artificial Cells
Researchers achieved timing-controlled DNA droplet division, enabling precise control of synthetic droplet dynamics, key to developing bio-inspired systems
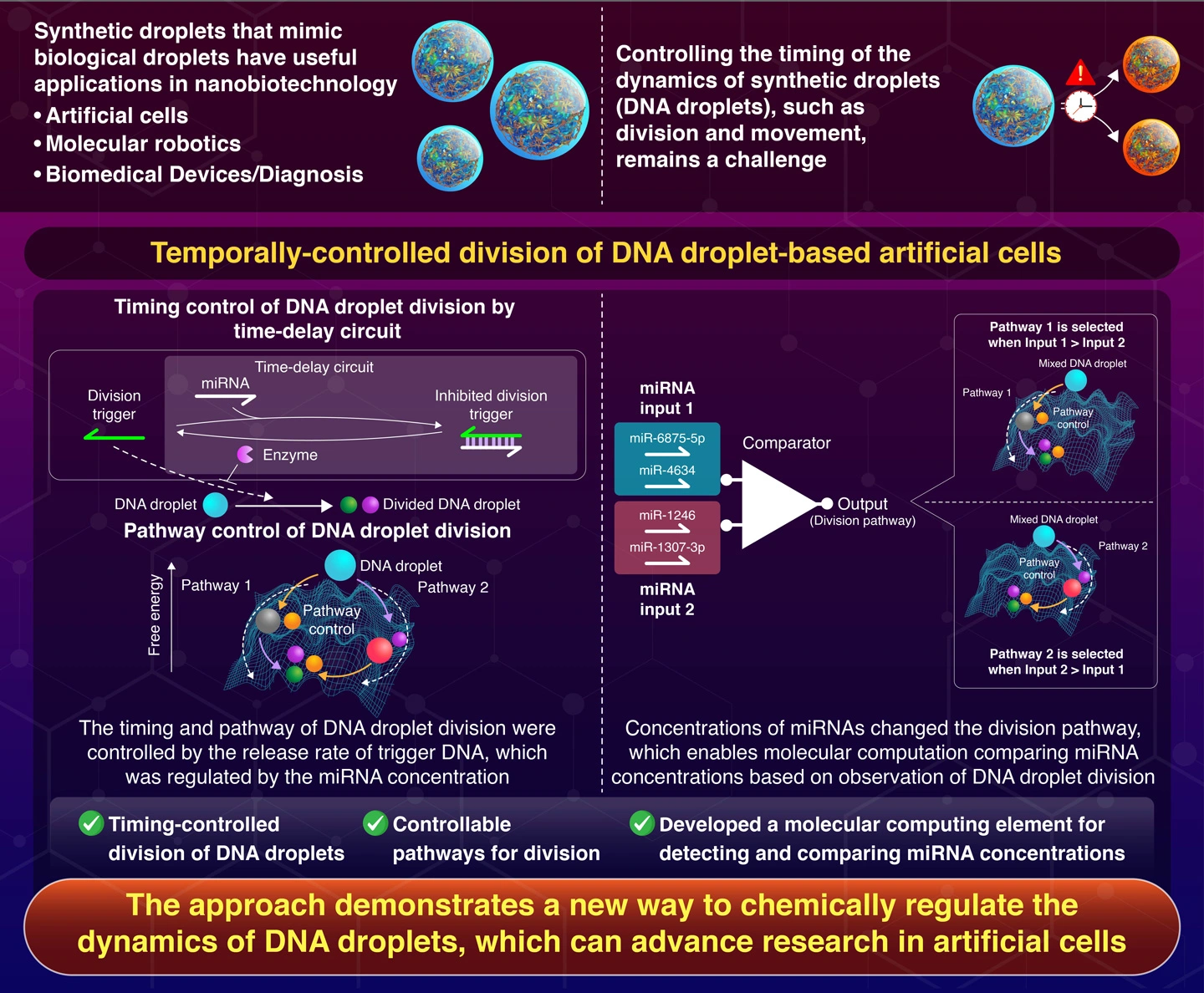
A time-delay circuit developed by researchers at Science Tokyo enables precise control over the division of synthetic DNA droplets, which mimic biological Liquid-Liquid Phase Separation (LLPS) droplets found in cells. By utilizing a combination of microRNAs (miRNAs) and the enzyme RNase H, the researchers have successfully regulated the timing of droplet division. This breakthrough paves the way for creating artificial cells with advanced functions, such as drug delivery and molecular computing.
Timing-Controlled Division of DNA Droplets for Molecular Computing and Nanobiotechnology Applications
Maruyama et al. (2024) | Nature Communications | 10.1038/s41467-024-51299-5
Many cellular functions in the human body are controlled by biological droplets called Liquid-Liquid Phase Separation (LLPS) droplets. These droplets, made of soft biological materials, exist inside living cells but are not enclosed by membranes like most cell structures. Because they lack membranes, LLPS droplets can adapt quickly to what the cell needs. They can move, divide, and change their structure or contents. This flexibility is essential for various functions, such as the transcription of ribosomal RNA (rRNA) in the nucleolus, enabling sol-gel transitions in which materials shift between fluid-like and gel-like states, and controlling chemical reactions within the cells.
Inspired by these unique properties, scientists have developed synthetic LLPS droplets to mimic their biological counterparts. While significant progress has been made in controlling the division and movement of synthetic droplets, precise control over the timing of these processes has remained a challenge.
A study published in the journal Nature Communications on August 27, 2024, marks a significant breakthrough in this field. Researchers from Institute of Science Tokyo (Science Tokyo), Japan, developed a method to precisely control the timing of division in synthetic DNA droplets, which mimic biological LLPS droplets. They achieved this by designing a time-delay circuit, where the division of droplets is regulated by a combination of inhibitor RNAs and an enzyme, Ribonuclease H (RNase H).
Professor Masahiro Takinoue, the lead author of the study explains: “We demonstrate the timing-controlled division dynamics of DNA droplet-based artificial cells by coupling them with chemical reactions exhibiting a transient non-equilibrium relaxation process, resulting in the pathway control of artificial cell division.”
In their approach, the DNA droplets are held together by Y-shaped DNA nanostructures linked via six-branched DNA linkers. These linkers can be cleaved by specific DNA sequences to the linkers used as division trigger DNAs. Initially, the division triggers are bound to single-stranded RNA (ssRNA) molecules called RNA inhibitors. Adding the enzyme RNase H degrades these inhibitors, freeing the division triggers to cut the DNA linkers and initiate droplet division.
“These two reactions cause a time delay in the cleavage of the DNA linker, resulting in the timing control of DNA droplet division” explains Takinoue.
The researchers successfully achieved pathway-controlled division in a ternary-mixed C·A·B-droplet system, consisting of three Y-shaped DNA nanostructures held together by two linkers. By inhibiting and controlling the release of division triggers, they established two distinct division pathways: Pathway 1, where C·A·B-droplets first divided into C-droplets and then A·B-droplets, and Pathway 2, where C·A·B-droplets initially divided into B-droplets and then C·A-droplets.
This pathway control was then applied to a molecular computing element known as a comparator, which compared concentrations of microRNA (miRNA) used as inhibitor RNAs. The comparator used differences in RNA concentrations to determine which pathway was followed, providing a method to quantitatively compare RNA levels, which has potential applications in diagnostics.
While the study’s chemical reactions showed promise, they were temporary and did not sustain a non-equilibrium state like cellular systems. To develop stable and sustainable non-equilibrium systems, researchers emphasize the need for chemical reactions that maintain a continuous supply of energy. Despite this, the research provides a valuable foundation for further advancements in controlling synthetic droplet dynamics.
“We believe that this technology provides a strategy to create artificial cells and molecular robots with more sophisticated functions, such as timing-controlled self-replication, drug delivery, and diagnosis, with more accuracy and quantitative specifications,” says Takinoue.
Reference
- Authors:
- Tomoya Maruyama1, Jing Gong1, and Masahiro Takinoue1,2,3*
- Title:
- Temporally controlled multistep division of DNA droplets for dynamic artificial cells
- Journal:
- Nature Communications
- Affiliations:
- 1 Department of Life Science and Technology, Institute of Science Tokyo, Japan
2 Department of Computer Science, Institute of Science Tokyo, Japan
3 Research Center for Autonomous Systems Materialogy (ASMat), Institute of Innovative Research, Institute of Science Tokyo, Japan
Further information
Professor Masahiro Takinoue
School of Computing, Institute of Science Tokyo
- takinoue@c.titech.ac.jp
Contact
Public Relations Division, Institute of Science Tokyo
- Tel
- +81-3-5734-2975
- media@ml.tmd.ac.jp